UNLOCK NATURAL OUTFLOW THROUGH THE UVEOSCLERAL PATHWAY


Finally, a new SURGICAL PATHWAY TO ENHANCE NATURAL OUTFLOW.
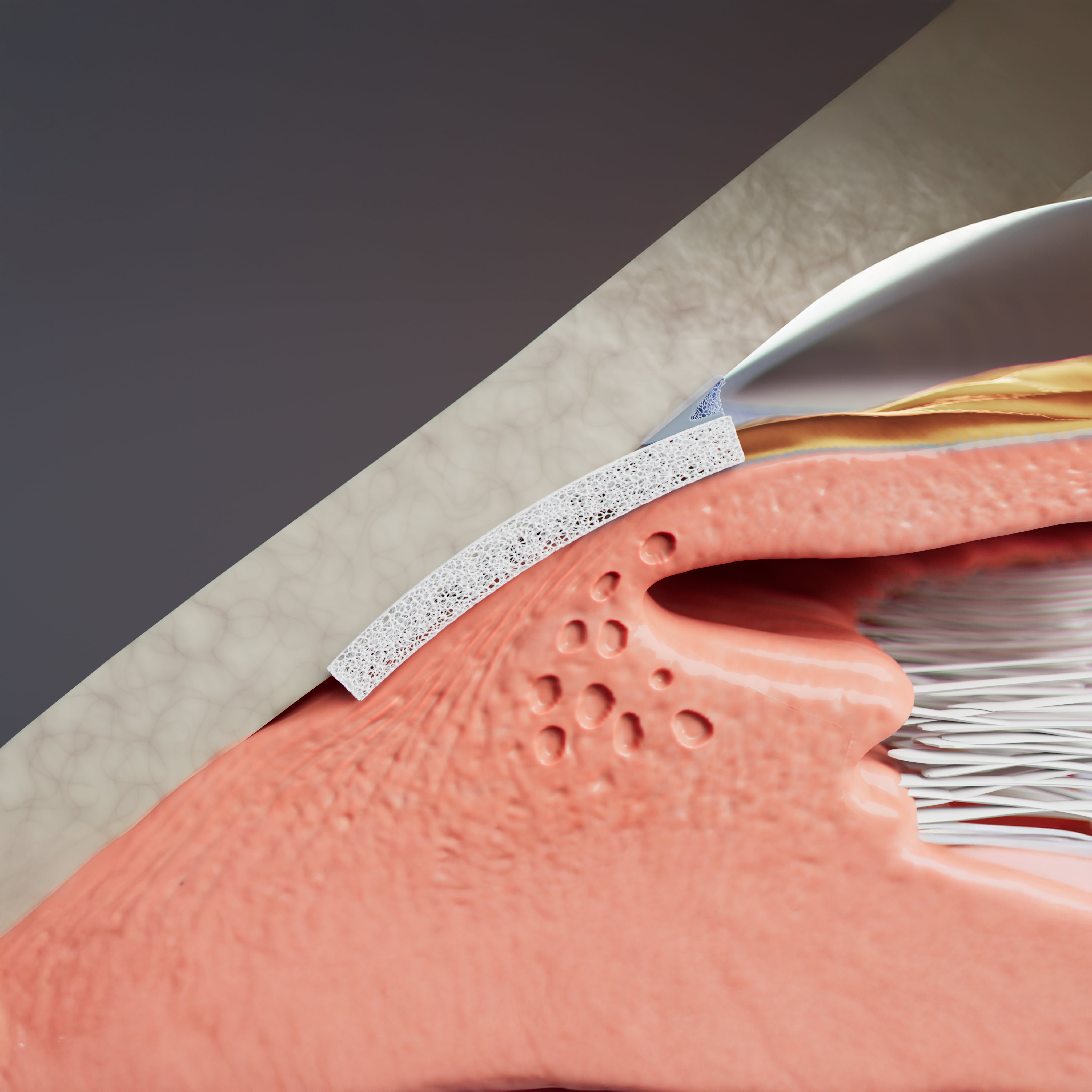
Maintains Aqeuous Pathway
A homologous bio-tissue for endoscleral reinforcement
Highly Permeable Bio-Tissue
Enhances uveoscleral aqueous outflow
Scleral Reinforcement
Scleral reinforcement without damage to the conjunctiva
Unlock the Other Half Of Natural Outflow
Work with your patient's natural physiology—while you stay in the angle
Tailored For the eye, from the eye.
Biologically Compatible
Acellular, minimizes risk of immune response.
Matches the native scleral structure.
Reduces risk of fibrosis and foreign body reaction.
Mechanical Strength and Durability
Collagen rich matrix resists degradation.
Easy to manipulate and highly conforming.
Non-biodegradable for long-term reinforcement.
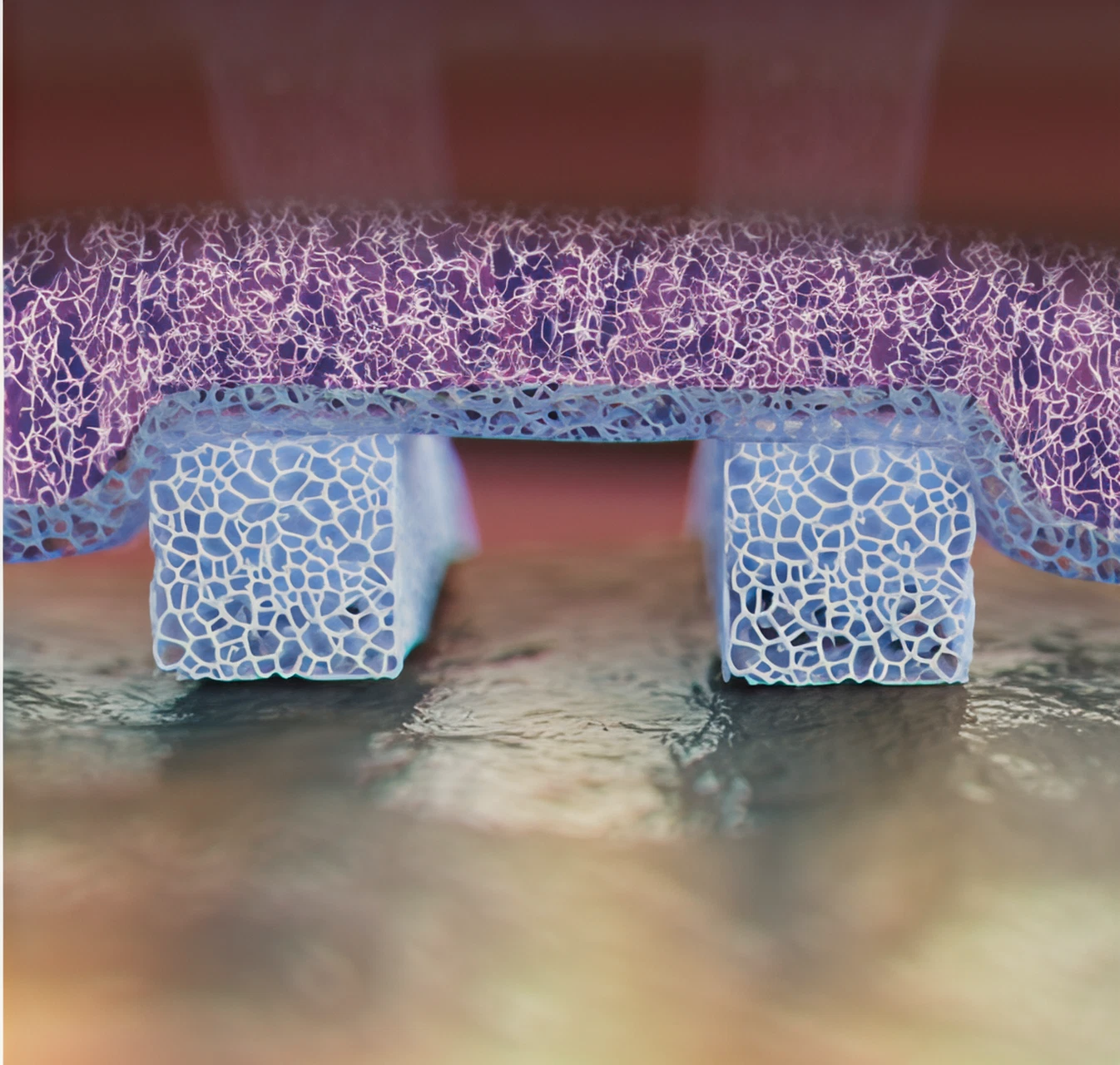
Facilitates Aqueous Conductivity
Naturally porous structure.
SEE WHAT SURGEONS ARE SAYING ABOUT ALLOFLO™ UVEO
Dr. Reena Garg, MD
Washington, DC


CONTROLLED CYCLODIALYSIS. PRECISE IMPLANTATION.
31 EYES
PUBLISHED PEER-REVIEWED RESULTS
mean IOP reduction1
mean medication reduction1
SERVING AN UNMET NEED IN YOUR CLINIC
Today there are over 2.5 Million eyes in the U.S. who’ve had trabecular outflow enhancing procedures and are experiencing waning efficacy —growing by 350,000 more each year.2
When the efficacy of surgical interventions plateau, higher-risk bleb-forming procedures have historically been the only remaining option. Not anymore.

RETURNS, REFUNDS & SHIPPING POLICY
In the U.S., faulty products may be replaced or credited at Iantrek’s discretion.
Returns must be requested within 30 days, with items unused and in original packaging. Refunds are issued after inspection; restocking fees may apply. Exchanges are available for defective or incorrect items.
Orders ship Monday–Friday. Report any delivery issues within 5 business days. Contact your Sales or Customer Service Representative for details.
PN: 900214 Rev A
